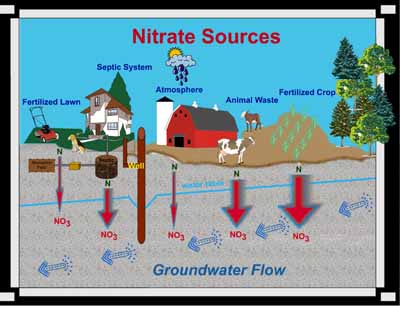
Nitrate (NO3) is a compound that is comprised of nitrogen and oxygen. Nitrogen comes from decomposing organic materials like manure, plants, and human wastes. Often the nitrogen (N) is derived from ammonia (NH3) or ammonium (NH4).
Plant species need nitrogen to form amino acids and proteins, which are essential for plant cell growth, but plants cannot use organic nitrogen directly. Microorganisms in the soil convert the nitrogen locked in crop residues, human and animal wastes, and compost to ammonium (NH4). Another specific group of microorganisms convert ammonium to nitrate (NO3), and since nitrate is water soluble, excess nitrate not used by plants can leach through the soil and into the groundwater.
The widths of the red arrows show relative
amounts of nitrate leaching
into groundwater.
into groundwater.
Nitrate is also present wherever biotic biproducts are breaking down or decomposing like animal waste, and septic system absorption fields or mounds.
On Friday, October 12th, our class took a trip out to the Municipal Wetlands in Springfield, Ohio for various sampling procedures; one of which involved the concentration of nitrate in the ground water. We used varying techniques of water acquisition depending on the state of the water (standing, flowing, or ground). For standing and flowing water, we collected a predetermined volume of water in vials. Once the sample was collected, a chemical indicator was added to produce a color reaction corresponding to a concentration of nitrate in the water of that particular locale. Using the color wheel on the measuring device, the specific color-concentration reading was obtained. For ground water samples, an additional step was needed to be performed before the chemical indicator was to be added. First, the area of interest was cored, allowing ground water to flow into the new opening. The water was collected, solid particles were allowed to settle out until the above testing procedures involving the indicator and color comparison were done.
Below is a video demonstrating the nitrate testing site as well as techniques used:
Surface, Standing Water:
Ground Water:
Why sample nitrate?
- Evan A.