Here we explore the Critical Zone that encompasses the lowermost groundwater to the atmosphere that meets the earth. Exchanges between rock, water, soil, and living things that are critical to our sustainability. To understand the importance of this zone to us, this blog will focus on Critical Zone processes in Springfield, Ohio, our home.
Monday, December 10, 2012
Reflections
Here are the students (Shirley, Emilie, Zach, Andrew, Stefan, Evan) who blogged so diligently about their insights into the Critical Zone. Last Friday they presented their posters describing their work in the Municipal Stadium Wetlands, Springfield, Ohio. This launches future community research projects by Critical Zone students. In their final presentation they described how local sites are ideal for ongoing student monitoring for scientific and learning reasons. They also suggested ways that we could further integrate the course into the community. (Zach suggested an afterschool science club exploration of the the Critical Zone mentored by undergraduates at Wittenberg). The research questions that remain to answer are still as simple as trying to understand the controls of biogeochemical change. What does this mean? We have a lot of exciting work ahead of us!
Saturday, December 8, 2012
Square Meter Challenge - The Dream Team
The Dream Team - Andrew Fuss, Evan Amstutz, and Zach Smith
For our square meter challenge, we chose to examine a portion of the naturally occurring wetland at the Municipal Wetlands in Springfield, Ohio. Our particular study site included approximately half dry land and half standing water. Included in the foliage were various species of riparian grasses, algal mats, and detritus material from surrounding maple and oak trees. At the deepest points, located furthest inland, the detritus and leaf litter extended 4.5cm down. The depth of the standing water gradually increased as measurements were taken farther away from land and reached a maximum depth of 6.0cm. When taking a soil core, the ground was understandably saturated with water and was comprised of primarily clay-based soil.
For our square meter challenge, we chose to examine a portion of the naturally occurring wetland at the Municipal Wetlands in Springfield, Ohio. Our particular study site included approximately half dry land and half standing water. Included in the foliage were various species of riparian grasses, algal mats, and detritus material from surrounding maple and oak trees. At the deepest points, located furthest inland, the detritus and leaf litter extended 4.5cm down. The depth of the standing water gradually increased as measurements were taken farther away from land and reached a maximum depth of 6.0cm. When taking a soil core, the ground was understandably saturated with water and was comprised of primarily clay-based soil.
Below is a video explaining our study site and the purpose of our study:
Below is a picture to get an idea of the scale of our study site:
- This is a dime that is laying on a piece of downed organic material
from a surrounding tree. It gives you an idea of the scale of our square
meter study site and its contents.
Below is a chart of the various measurements we made describing the chemical and physical characteristics of the study site:
With these small scale observations, we can make certain assumptions about the rest of the wetland area. Assuming that the surrounding area behaves in a similar fashion to the square meter study site, we can extrapolate our readings to the rest of the wetland. This is a valuable tool for studying a location with a relatively large area.
- Andrew Fuss, Evan Amstutz, and Zach White
Friday, December 7, 2012
Concept Mapping of Geologic Processes
Our Concept Map:
The three
cycles shown in the concept map, the nitrogen cycle, the phosphate cycle and
the carbon cycle are all different and important in their own way, being major
categories with numerous inter-relations, as the concept map shows. The
nitrogen cycle is the process by which nitrogen is converted between its
various chemical forms. This transformation can be carried out through both
biological and physical processes. Important processes in the nitrogen cycle
include fixation, mineralization, nitrification, and dentrification. Also,
human activities such as fossil fuel combustion, use of artificial nitrogen
fertilizers, and release of nitrogen in waste water have dramatically altered
the global nitrogen cycle. The phosphate cycle is important, because it is
essential to both plants and animals, which includes humans, because of their
importance in terms of developing healthy seeds, root growth, and stem strength
for plants and developing healthy bones (works with Ca to build bone tissue)
for animals (humans). Phosphorus is released from rock into the soil by a
process called weathering. In land phosphorous is cycled by plants which take
up phosphate through their roots, animals who eat the plants (get phosphate)
and decomposers who return it to the soil. Phosphorous also gets cycled through
our waterways by getting into the water by erosion, leaching, run-off with most
settles at the bottom (turns into sediment), while some phosphate is taken up by
aquatic plants. Humans affect the P cycle in a number of ways, such as, mining
phosphate rock (for fertilizers and detergents),making fertilizers and
detergents (industrial waste), applying fertilizer to land and by fishing. The
carbon cycle is the biogeochemical cycle by which carbon is exchanged among the
biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. The
carbon cycle comprises a sequence of events that are key to making the Earth
capable of sustaining life; it describes the movement of carbon as it is
recycled and reused throughout the biosphere. Human activity has modified the
carbon cycle by changing its component's functions and directly adding carbon
to the atmosphere, with the largest and most direct human influence on the
carbon cycle being through direct emissions from burning fossil fuels, which
transfers carbon from the geosphere into the atmosphere.
We
hypothesize that the concentrations of nitrate and phosphate (both found in
fertilizer) will decrease the further into a state nature reserve (like the
ones around here, surrounded by farmland) one tests using core samples. We
cause anthropogenic changes (an example of a forcing) to the soil of the nature
preserve, which is shown in both our conceptual map and the Brantley conceptual
map, which contributes to our hypothesis. These additions of nitrate and
phosphate from fertilizing of farmland causes change to the wildlife presence
in the wetlands. This change of the wildlife presence causes alterations to the
carbon cycle, by causing a change in the plant population, which directly
sequesters carbon dioxide. We expect that our wetland will respond similarly if
not in the same way as other wetlands that are surrounded by farmland. We would
also expect that our wetland will have a more exaggerated response to fluxes of
nitrate and phosphate, characteristic of a small scale wetland.
- Stefan Latham, Evan Amstutz, and Andrew Fuss
Thursday, December 6, 2012
Concept Mapping in our Geology 170 Class
Concept Mapping Main Concepts in Geology 170
We categorized our concept map into 5 categories. They were large scale, measurements, cycles, observations,and small scale. We found that these were the most important categories because of their importance in the critical zone. The large-scale category is comprised of the world, National (USA), photosynthesis, etc. The measurements category was comprised of tools we used in the field, for example, YSI instrument, pH testing, phosphate testing, etc. Our cycles category was comprised of the rock cycle, phosphate cycle, water cycle, nitrogen cycle, and the carbon cycle. Our next category, observations, was comprised of things we actually saw or interacted with while out in the field. Our last category, small scale, was comprised of Ohio, Springfield, and Buck creek. All of these categories are inter-related because without one of the other categories,everything wouldn't function as it does.
What does a concept map look like?
Want to see our concept map? Click this link:)
http://prezi.com/vuo8p3zc2n_w/concept-mapping/?kw=view-vuo8p3zc2n_w&rc=ref-25775691
Linking the Concept map of Brantley and Ours:
In comparing both concept maps, we found that we could study the new hypothesis that we can test the linkage between the community and how the levels of phosphate and nitrate. His concept map shows the anthropogenic issues ( human effect). Our concept shows the observations that humans could effect. System thinking is useful for science because it can connect multiply concepts to solve issues.
Blog by: Shirley, Emilie, Zach
We categorized our concept map into 5 categories. They were large scale, measurements, cycles, observations,and small scale. We found that these were the most important categories because of their importance in the critical zone. The large-scale category is comprised of the world, National (USA), photosynthesis, etc. The measurements category was comprised of tools we used in the field, for example, YSI instrument, pH testing, phosphate testing, etc. Our cycles category was comprised of the rock cycle, phosphate cycle, water cycle, nitrogen cycle, and the carbon cycle. Our next category, observations, was comprised of things we actually saw or interacted with while out in the field. Our last category, small scale, was comprised of Ohio, Springfield, and Buck creek. All of these categories are inter-related because without one of the other categories,everything wouldn't function as it does.
What does a concept map look like?
| This is an example of a concept map |
http://prezi.com/vuo8p3zc2n_w/concept-mapping/?kw=view-vuo8p3zc2n_w&rc=ref-25775691
Linking the Concept map of Brantley and Ours:
In comparing both concept maps, we found that we could study the new hypothesis that we can test the linkage between the community and how the levels of phosphate and nitrate. His concept map shows the anthropogenic issues ( human effect). Our concept shows the observations that humans could effect. System thinking is useful for science because it can connect multiply concepts to solve issues.
Blog by: Shirley, Emilie, Zach
Saturday, December 1, 2012
Square Meter observations of Municipal Wetland
Our Challenge~
Overall:
Overall, with our observations we can predict how the rest of the wetland would react to certain conditions, and how the levels of nitrate and phosphate are in the area. In doing small-scale observations we can predict things on a larger scale and then carry out larger scale operations.
- We had to make as many observations within one square meter of the Municipal Stadium Wetland. In doing so we would use our field tools that we have learned such as; YSI meter testing, pH testing, Nitrate, Phosphate, and temperature readings. In our challenge we chose a site and then squared it off. Then recorded as much data as possible.
Our Site:
Our Purpose: This video explains our purpose for taking these observations:
Our Observations:
- In looking at this site, we saw that the area was 50% under mucky water and the other 50% was not. We also measured the level of vegetation, the vegetation in this location was 47cm above the ground. Then once we recorded the visible observations, we started to record more of the data that involved testing kits, and material.
- In our observations we also compared something that we found there with a coin. Also we observed a spider on a twig.
 |
| In the red circle is a spider on a twig. |
 |
| In the red circle is the coin, as you can tell the scale of our observations were larger than the coin. |
Our Results:
 |
| This is our collected data that we had within our meter by meter location |
Overall, with our observations we can predict how the rest of the wetland would react to certain conditions, and how the levels of nitrate and phosphate are in the area. In doing small-scale observations we can predict things on a larger scale and then carry out larger scale operations.
by: Emilie, Shirley, and Stephan
Wednesday, November 28, 2012
Phosphate - Scale
With the data that we have collected you are able to look at
the behavior between time and scale of the Ohio River averages in the month of
September as well as Buck Creek and we then are able to look at the results
between the two. The Ohio River averages are [7g/km2/h], unlike that of Buck
Creek, which is at (0.62g/km2/h).
When looking at the data it is needed to be understood that
the Phosphate was collected at Buck Creek over a one day period. During this
day, many samples were taken at the site. There were two samples that were
collected at each of the 5 locations in the watershed we were studying, for a
total of 10 samples from the watershed.
Upper Miami Watershed (http://www.epa.state.oh.us/dsw/tmdl/GreatMiamiRiver.aspx)
The Ohio River data was collected over a span of 12 months,
for a 48 year time period from 1963-2011. Every sample was taken at the
beginning of the month. With those samples that were taken, we then just
focused on the month of September. The data was then looked at for every
September in the time period.
In conclusion, of all the data gathered from Buck Creek and
the other data collected from the Ohio River, one can conclude that the
differences in the scales vary due to size, space, location, and time of the
collection period in the different watersheds.
- Zach Smith & Stefan Latham
Sunday, November 18, 2012
Nitrate Behavior - A Scalar Comparison
Mississippi River Watershed (rocketcare.utoledo.edu)
In most cases of excess nitrate concentrations in aquatic systems, the primary source is surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. This process of excess fertilizer accumulating in aquatic ecosystems is called eutrophication and can lead to the sudden appearance of algal blooms. Because of the quick onset of floral growth, the ecosystem can develop water anoxia and dead zones, as well as the blooms causing other changes to ecosystem function, favoring groups of organisms better suited for the low oxygen content. Effectively, the excess of nitrate can change the makeup of aquatic biodiversity.
Upper Great Miami Watershed (epa.state.oh.us)
Fluctuations in dissolved nitrate present in waterways can vary depending on the scale of comparison. Within the small streams and rivers of Ohio,fluctuations in nitrate concentration may not be the most extreme. When looking at the data provided by the USGS concerning the Upper Great Miami watershed, we observed a small fluctuation in the minimum and maximum levels of nitrate being transported downstream (measured in g/km/hr). Below is a graph of the range (lowest to highest) and average hourly-area normalized yield of total N and total P for the Ohio River during the month of September based on all September averages:
When observing the fluctuations in the levels of dissolved nitrate transport in the waterways that the Upper Great Miami drain into, like the Ohio and Mississippi river, differences in the maximum and the minimum measurements differ much more drastically. The measurement of these waterways differ in this way because these large aquatic systems move water that had ran off of a much greater area of land. The amount of dissolved nitrate present is a result of many watersheds' water output compounding to create a highly concentrated volume when finally reaching the Mississippi River Delta and dumping into the Gulf of Mexico.
- Evan Amstutz
Tuesday, November 13, 2012
Phosphate Behavior Comparison
In looking at the
behavior between scales (time/scale) we discovered that the Ohio River averages
over the month of September was [7g/km2/h], unlike that of Buck Creek, which is
less than [1g/km2/h], at (0.62g/km2/h).
By looking at the
comparison of data, Phosphate collected at Buck Creek was collected over a time
period of 1 day. In that 1 day, we took two samples at every 5 spots within our
location in that watershed.
 |
| Ohio WaterShed |
 |
| Buck Creek WaterShed |
The data of the
Ohio River was over a span of 12 months, with samples being taken on the first
of each month from 1963- 2011 (48 years). When we narrowed that data down, we
chose the month of September. We had data from the 1st of September
for the years of 1963-2011.
So, from both our
own collection of data from Buck Creek, and the data that we found on the Ohio
River we can conclude that the differences in the scales vary due to size/space
and time of the collection period. ( sources for pictures Google maps)
By : Shirley and Emilie
By : Shirley and Emilie
Thursday, October 25, 2012
Testing Nitrate Levels in the Municipal Wetlands
What is Nitrate?
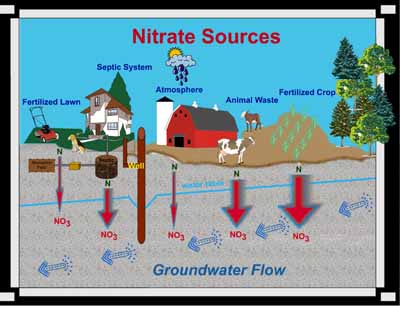
Nitrate (NO3) is a compound that is comprised of nitrogen and oxygen. Nitrogen comes from decomposing organic materials like manure, plants, and human wastes. Often the nitrogen (N) is derived from ammonia (NH3) or ammonium (NH4).
Plant species need nitrogen to form amino acids and proteins, which are essential for plant cell growth, but plants cannot use organic nitrogen directly. Microorganisms in the soil convert the nitrogen locked in crop residues, human and animal wastes, and compost to ammonium (NH4). Another specific group of microorganisms convert ammonium to nitrate (NO3), and since nitrate is water soluble, excess nitrate not used by plants can leach through the soil and into the groundwater.
Nitrate is also present wherever biotic biproducts are breaking down or decomposing like animal waste, and septic system absorption fields or mounds.
On Friday, October 12th, our class took a trip out to the Municipal Wetlands in Springfield, Ohio for various sampling procedures; one of which involved the concentration of nitrate in the ground water. We used varying techniques of water acquisition depending on the state of the water (standing, flowing, or ground). For standing and flowing water, we collected a predetermined volume of water in vials. Once the sample was collected, a chemical indicator was added to produce a color reaction corresponding to a concentration of nitrate in the water of that particular locale. Using the color wheel on the measuring device, the specific color-concentration reading was obtained. For ground water samples, an additional step was needed to be performed before the chemical indicator was to be added. First, the area of interest was cored, allowing ground water to flow into the new opening. The water was collected, solid particles were allowed to settle out until the above testing procedures involving the indicator and color comparison were done.
Nitrate is a common contaminant found in many wells, wetlands, rivers, and other waterways. Shallow wells, wetlands, and streams in close proximity to agricultural land, and that collect runoff from cultivated land are the most vulnerable to nitrate contamination. Major sources of nitrate contamination can be from fertilizers, animal waste, and human sewage. The Environmental Protection Agency highly recommends testing drinking water supply on a regular basis. Elevated levels of nitrate in drinking water can cause Blue Baby Syndrome in infants under six months of age and that are bottle fed. Long term health effects to older children and normal healthy adults exposed to elevated levels of nitrate in their drinking water are not yet agreed upon in the scientific community. However, the National Cancer Institute suggests a link between elevated levels of nitrate in drinking water and an increased risk of non-Hodgkin's lymphoma (a cancer of the lymphatic system).
- Evan A.
Nitrate (NO3) is a compound that is comprised of nitrogen and oxygen. Nitrogen comes from decomposing organic materials like manure, plants, and human wastes. Often the nitrogen (N) is derived from ammonia (NH3) or ammonium (NH4).
Plant species need nitrogen to form amino acids and proteins, which are essential for plant cell growth, but plants cannot use organic nitrogen directly. Microorganisms in the soil convert the nitrogen locked in crop residues, human and animal wastes, and compost to ammonium (NH4). Another specific group of microorganisms convert ammonium to nitrate (NO3), and since nitrate is water soluble, excess nitrate not used by plants can leach through the soil and into the groundwater.
The widths of the red arrows show relative
amounts of nitrate leaching
into groundwater.
into groundwater.
Nitrate is also present wherever biotic biproducts are breaking down or decomposing like animal waste, and septic system absorption fields or mounds.
On Friday, October 12th, our class took a trip out to the Municipal Wetlands in Springfield, Ohio for various sampling procedures; one of which involved the concentration of nitrate in the ground water. We used varying techniques of water acquisition depending on the state of the water (standing, flowing, or ground). For standing and flowing water, we collected a predetermined volume of water in vials. Once the sample was collected, a chemical indicator was added to produce a color reaction corresponding to a concentration of nitrate in the water of that particular locale. Using the color wheel on the measuring device, the specific color-concentration reading was obtained. For ground water samples, an additional step was needed to be performed before the chemical indicator was to be added. First, the area of interest was cored, allowing ground water to flow into the new opening. The water was collected, solid particles were allowed to settle out until the above testing procedures involving the indicator and color comparison were done.
Below is a video demonstrating the nitrate testing site as well as techniques used:
Surface, Standing Water:
Ground Water:
Why sample nitrate?
- Evan A.
Wednesday, October 24, 2012
Wetlands
The Municipal Stadium Wetlands are a very important resource
for the Springfield and Wittenberg communities, as well as, a very beautiful,
peaceful and idyllic location that can be enjoyed by the Springfield and
Wittenberg communities. The Springfield community has certainly taken notice of
the Wetlands importance with a recent article in the Springfield News Sun,
about the wetlands and their value, in terms of a wetlands capacity to capture
pollutions and impurities. A link to the article mentioned is http://www.springfieldnewssun.com/news/news/local/stadium-wetland-keeps-creek-idea-alive/nSZ8q/.
However, as an individual who was born and raised in Springfield, I hope
that this beautiful and educational place can be maintained and kept clean and
welcoming, rather than attracting negativity. The Municipal Stadium Wetlands
must be maintained as a positive place, that allows positive individuals to gather
their without feeling unsafe or wary of their surroundings, because it is such
an important resource, as well as a very pleasant place where people can enjoy
nature.
-Stefan Latham
Saturday, October 20, 2012
Testing pH levels and Temperature in our Wetland
Our Mission at the Wetland!
~ Our wetlands provide us with diversity of animal species and protect and filter our water. In the efforts to learn more about them, we have been collecting data of the pH levels of the water and also the temperature to see how this is related to the critical zone. By learning more we are able to take action and protect our wetlands. We tested the temperature using a YSI prob( which is described in the video below).
(source: youtube.com)
source: youtube.com
~What is pH?
pH is defined as: A measure of the acidity or alkalinity of a solution, numerically equal to 7 for neutral solutions, increasing with increasing alkalinity and decreasing with increasing acidity. The pH scale commonly in use ranges from 0 to 14. ( dictionary.com)
~ Our wetlands provide us with diversity of animal species and protect and filter our water. In the efforts to learn more about them, we have been collecting data of the pH levels of the water and also the temperature to see how this is related to the critical zone. By learning more we are able to take action and protect our wetlands. We tested the temperature using a YSI prob( which is described in the video below).
(source: youtube.com)
~What is pH?
pH is defined as: A measure of the acidity or alkalinity of a solution, numerically equal to 7 for neutral solutions, increasing with increasing alkalinity and decreasing with increasing acidity. The pH scale commonly in use ranges from 0 to 14. ( dictionary.com)
Video explaining pH:
(Source: youtube)
Us testing pH levels at Wetland:
~What is Temperature?
Temperature is defined as:a measure of the warmth or coldness of an object or substance with reference to some standard value. The temperature of two systems is the same when the systems are in thermal equilibrium. ( Temperature is very important to test because if the water is too hot then some species may not survive in the conditions. For example: in Buck Creek where we tested first, it is know for the trout because the water is at a cooler temperature.)
Testing Temperature:
Overall Summation:
~ All these tests are very important in order to preserve and protect our wetlands.
Testing Temperature:
Overall Summation:
~ All these tests are very important in order to preserve and protect our wetlands.
Municipal Stadium Wetland, Springfield Ohio
 |
| Probing wetland prior to sampling. |
Dr. John Ritter and his students have installed instrumentation in the wetland to monitor changes in water stage, pH, ORP, temperature, and turbidity (See Fact Sheet). There is interest in the site from the City of Springfield Wetland Article by Tom Stafford
While the size of the wetland is small, nutrient (fertilizer) management continues to be a huge problem for the Mississippi River Basin. Low-cost mitigation options might include targeting riparian areas of streams like Buck Creek for additional innundation and wetland creation. This site is ideal for study because watersheds like the Upper Great Miami that have among the highest nutrient loads (Combined Sewage Overflow & agricultural runoff).In fact, our class plotted data from a recent USGS study showing that the Great Miami is in the top 30 N and P loading watersheds of 800 that feed the Mississippi River basin and in the top 5 for watersheds in Ohio.
Geology of the Critical Zone is conducting a baseline study of the nitrate and phosphate concentrations in the wetlands during the drier period of the early fall. An upper-level projects class will conduct temporal sampling during the Spring Semester when the wetland will flood. This class will expand investigations of nitrate and phosphate and also examine chemical weathering (and its role in climate change). It is relatively unknown how mineral weathering is altered by the presence of wetlands, and yet this process regulated the earth's temperature over geologic timescales.
Friday, October 19, 2012
Sampling Wetland Pore Water
Water added to the soil by rainfall or irrigation percolates downward to groundwater unless it runs off to surface waters, evaporates, is taken up by plants, or remains within the soil profile Chemicals such as fertilizers or pesticides can move with the water if they are not first broken down into other chemicals, transformed into gases, retained by chemical interaction with the soil solids, or taken up by plants or soil organisms. Successful crop production depends on careful management of soils, water, and chemicals so that plant needs are met as they occur in the growing season. Meeting these needs efficiently may also help to protect the quality of underlying groundwater by reducing the amount of chemicals being carried downward by recharge waters.Water in the soil originates from precipitation, irrigation, or upward flow from groundwater in areas with shallow water. It can contain dissolved minerals derived from the soil or atmosphere, as well as soluble pesticides, fertilizers, and other chemical compounds used or disposed of at the land surface. When soils are not saturated with water, then the pores also contain a mixture of gases, including nitrogen, oxygen, and carbon dioxide (as in normal air) and more exotic types such as methane, phosphate and hydrogen sulfide. Soil gases are produced and assimilated by soil organisms, plant roots, and decay processes, and they are exchanged with gases from the atmosphere. Without adequate exchange of gases in soil pores, crop growth cannot occur because the oxygen needed by the plant roots would rapidly become depleted. Most water management in soil is aimed at providing sufficient water for plants without producing conditions of excess water that prevent proper gas exchange.
The link above is the link for the video of sampling the nitrate in the water from the pores in the wetland in Springfield, Ohio. The links below support that.
http://www.water.ncsu.edu/watershedss/info/wetlands/function.html
http://water.epa.gov/type/wetlands/assessment/survey/upload/2007_09_17_wetlands_survey_Rick_Savage_NWMAWG_KansasCity.pdf
http://www.es.govt.nz/environment/land/wetlands/waituna/soil/
Zach Smith
The link above is the link for the video of sampling the nitrate in the water from the pores in the wetland in Springfield, Ohio. The links below support that.
http://www.water.ncsu.edu/watershedss/info/wetlands/function.html
http://water.epa.gov/type/wetlands/assessment/survey/upload/2007_09_17_wetlands_survey_Rick_Savage_NWMAWG_KansasCity.pdf
http://www.es.govt.nz/environment/land/wetlands/waituna/soil/
Zach Smith
Monday, October 15, 2012
Methods of Soil Creation
All around the world, the material that lies beneath us is crucial to what can grow and what can be built in a given area. Especially important to the farming community is the general makeup of the soil to be planted on. Its presence is often taken for granted and its origin often overlooked. From varying compositions of parent material (the original rock that gets broken down), soils of many types and mineral contents are formed. Soil forms by three non-mutually exclusive methods:
1). Physical/Mechanical: methods that involve a mechanical breaking or grinding of the parent material. Ex. - rainfall, glacial movement, wind exposure, water freezing (seen below), etc.
1). Physical/Mechanical: methods that involve a mechanical breaking or grinding of the parent material. Ex. - rainfall, glacial movement, wind exposure, water freezing (seen below), etc.
(/www.seafriends.org.nz/enviro/soil/geosoil.htm)
2). Chemical: methods that involve the alteration of parent rock by the effects of the presence of chemicals on the rock surface. Ex. - acid rain, pollutant spills, runoff, etc.
3). Biological: methods that involve biota effecting the rate of the breaking down of the parent rock. Ex. - root growth into rock, growth on the rock surface altering the level of exposure, biproducts of life processes causing deterioration of rock surface, etc. These can also be considered physical or chemical, but since they are due to an organisms life processes, they are categorized as biological methods.
The balance between soil formation and soil erosion has been both positively and negatively impacted by the presence of human activity. The urbanization of many areas has slowed the progress of the natural cycle of soil production and erosion by placing a barrier (a building, parking lot, etc.)between the soil and the environment. Also, the over-farming of areas has sped up the process of soil erosion and nutrient-leaching. With over-farming, the root growth also breaks up the soil faster than what occurs under natural conditions. Despite these negative impacts, the presence of humans has led to the preservation of many areas of natural soil production and erosion by the implementation of legislation defining nature preserves and state/national parks.
One of the most heavily affected areas of negative human impact on soil production and erosion is on the island nation of Haiti. Here, the lumber trade has cut down a large majority of the nation's trees, leaving the ground underneath without a support structure that was originally supplied by the complex tree root system. Because of the lack of support, the soil is washing down hill, carrying sewage, trash, and more importantly homes. Fresh water is then heavily silted and polluted and unsuitable for human use. The washing away of soil has also left the agricultural community without a place to plant their crops.
Soil, both in Haiti, and also elsewhere in the world is important to preserve because of the geologic timescale in which it is formed. When we destroy the quality of soil now, we neglect the time required to replenish a suitable soil composition for our uses. It takes tens of thousands of years for parent rock to develop into soil through the three processes discussed above. It only takes a matter of years for us to decimate an entire nation's soil supply and utilization, but it will take an untold amount of time to recover.
- Evan A.
Tuesday, October 9, 2012
Soil Formation
Soil is formed from the weathering of rocks and minerals. The surface rocks break down into smaller pieces through a process of weathering and is then mixed with moss and organic matter. Over time this creates a thin layer of soil.
http://skywalker.cochise.edu/wellerr/students/soils/soils_files/image002.gif
Human disturbances can affect the formation and erosion of soil greatly. Urbanization greatly affected soil erosion because to create buildngs we need to get rid of the land to build on it. Agriculture also affects soil erosion by planting crops on it and changing the terrain. Finally, deforestation affects soil erosion because humans remove the land.
The haiti problem is from deforestation. The cutting down of trees has changed their country. The quest for wood decimates the forest. The damaged caused kills people, removes homes and also affects their water quality. This water affects hundreds of thousands of people due to this cycle of destruction. Soil is very important to the world because we are losing much more soil than what we are forming. If it takes thousands of years to create small amounts of soil, eventually we will not have any more soil. This can affect our agricultural aspect of the world.
-Andrew Fuss
http://skywalker.cochise.edu/wellerr/students/soils/soils_files/image002.gif
Human disturbances can affect the formation and erosion of soil greatly. Urbanization greatly affected soil erosion because to create buildngs we need to get rid of the land to build on it. Agriculture also affects soil erosion by planting crops on it and changing the terrain. Finally, deforestation affects soil erosion because humans remove the land.
The haiti problem is from deforestation. The cutting down of trees has changed their country. The quest for wood decimates the forest. The damaged caused kills people, removes homes and also affects their water quality. This water affects hundreds of thousands of people due to this cycle of destruction. Soil is very important to the world because we are losing much more soil than what we are forming. If it takes thousands of years to create small amounts of soil, eventually we will not have any more soil. This can affect our agricultural aspect of the world.
-Andrew Fuss
How is Soil Formed?
~ How is soil Formed?~
3 Factors Human disturbances of Soil:
~~~ The formation of soil starts with the parent material (earthy
materials such as minerals or organic material) the parent material is then
broken down into small particles by the process of weathering. The process is
controlled by the climate of an area (temperature, humidity, rainfall,etc.).
Also both plants and animals help soil form. As these
organisms die they add organic matter to weathered parent material to help form
topsoil and subsoil( aka the layer beneath topsoil). Also these animals/ plants decompose they build layers.
Another factor that helps form soil is the topography (the
hilliness, flatness, or amount of slope of the land). The other portion of the
soil forming process is time. The age of soil takes hundreds of years for all
these factors to form on inch of soil from the parent material.
Doesn't make sense? Well here is a short video that explains it with a visual :) Also here is a photo of how soil may look beneath the surface.
( source:http://www.youtube.com/watch?v=vg-hwKwT-Hs&feature=related) I edited it to the soil part)
 |
| Emilie Naccarato's hands during a soil sample of our wetland site ( source Dr. Fortner's camera) |
Human activities are a major disturbance in the balance between soil formation and soil erosion.
Industrial farmland is very vulnerable to erosion because of intensive tillage, which is
plowing(the displacement of the ground). This tillage gets rid of protective ground cover from the
soil surface, as well as damages root systems, which help hold the soil together. Nutrient cycles
are a little different on farms. Due to the constant crops being harvested and eaten by the
livestock and humans ,no continual supply of decaying plant material to replace nutrient levels
within the soil. This causes nutrients to be replenished by the addition of fertilizers in the soil.
Also, the restoration of organic matter to the soil by synthetic fertilizers has been presented to
negatively affect soil productivity. Long-term depletion of organic matter is caused by these
fertilizers. Important minerals such as calcium, magnesium, and potassium that leach out of the
soil are the result of over fertilization. These nutrients in large concentrations become harmful to
organisms within the soil. (http://www.sustainabletable.org/issues/soil/) Lastly, interaction amongst humans
and soil erosion through building and mining is an adverse process of soil formation. The use of
soil is the basis for societies and our soil is basically nonrenewable within the human time scale.
We have used it in ways that are not part of the natural process http://webcache.googleusercontent.com/search?q=cache:mP_UMOO8oZwJ:www.public.iastate.edu/~jasandor/Human%2520Impacts%2520on%2520Soil%2520Formation%2520Final.doc+soil+formation+AND+human+activities&hl=en&gl=us
The Haitian Situation:
~ A) The source of the Haitian situation is the deforestation( the cutting down of trees). These trees normally protect topsoil by catching the rain and slowing its fall to the ground. The other sources are: erosion, flash-foods pollution, and increased salt concentrations. With all these problems it decreases the way soil normally functions.
B) The effects of these massive lands shifts are those of the explainable Towns are harmed by the massive mud slides and flash floods With trees being cut down there is nothing for the water to slow down. Then the chemicals in fertilizers used on farms run into water sources killing organisms.
C) Our soil is important to protect because it protects our ecosystems. With great quality of soil, our crops will produce faster and better and have more nutrients.
Monday, October 8, 2012
soil forming
Soil is made of minerals, decaying matter, water and air. Living things die and start decaying. As they break apart into bits, wind, water and other natural processes mix this up with minerals already in the ground adding air and water. This process takes a great amount of time to occur. That is how soil is made. As this material becomes exposed to the surface they begin to erode and become altered chemically. The type of soil that forms is a result of the available rock, and which minerals are contained within. Sandstone will form coarse sandy soil. Soft shale will turn into heavy clay soils and granite bedrock produces a sandy loam.
Humans have a huge impact on the removal of soil but not as much of an effect on the production of soil. The difference between those two things are great. Humans destroy soil in many different ways a couple examples of this is agriculture, deforestation, over-grazing, construction, and
mining. These things can lead to the erosion of soil.
Hatian situation
A) The erosion is from the the deforestation from the trees being cut down for charcol production. Once the trees are cut down the water from the ground and the rain take the soil and the nutrients from the soil tumbing down the mountain side, (losing all chance for reforestation due to the nutrients being washed away) not having anything there to stop the soil but the houses below.
B)The damaged caused by these massive shifts of land are terrible. First if there is a town or village below the mountain then the people of that place have no where to go as the soil comes down destroying and flatening everything in its path. Also the runoff gets into to rivers and lakes causing the aquatic animals of that area to die due to the flooding of nutrients and all of the soil being thrown into the water that fast.
C)Protecting the quality of our soils is as important as protecting the air we breathe and the water we drink. Protecting soil is critical to protecting our ecosystems and our ability to raise crops or maintain a backyard garden. Soil quality can be a measure of soil productivity. Soil quality can also be linked to water quality. If we protect the water we drink and the air we breathe then why don't we protect the soil which can have a direct effect on both water and the air depending on what materials get into the soil.
Zach + Stefan
Subscribe to:
Comments (Atom)